Process solutions for biotech and pharmaceuticals
We don’t just adapt to your needs and develop tailor-made solutions for you – we also produce individual equipment in-house, including containers or isolators, enabling us to provide you with an ideal overall concept. The Waldner Process Systems’ product range is broad-based, meeting all your individual applications:
- Production and processing of active and highly active pharmaceutical ingredients (API and HAPI)
- Production and processing of cytostatics for chemotherapy drugs
- Vaccine production
- Processing of blood plasma
- Buffer and media batching
- Preparation of aseptic liquid forms (infusion solutions / parenteralia)
- Cleaning processes (Clean in place CIP / Sterilise in place SIP)
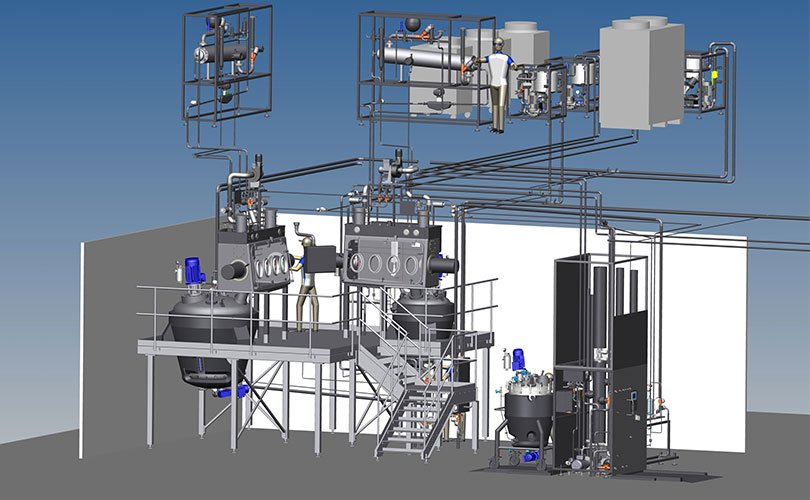
Designing the systems

Our engineers and technicians work together at the start of a project to develop high-quality and integrated process solutions. Their project experience and specific knowledge enable them to design the system to optimise productivity and maintenance, which, in turn, is also optimised for users – for maximum cost-effective and technical safety of users. System production and peripherals are optimised, with skids and package units ensuring a quick assembly and a safe commissioning process.
Products
Our core expertise also includes the batching of liquid products or buffer solutions, if required in conjunction with our isolators or a suitable solids handling unit. We also specialise in the mixing and handling of high-viscosity or aseptic products.

Boilers, containers, reaction vessels and pipework must be capable of being cleaned in place and without disassembly, wherever there are critical hygiene requirements. Clean-in-place systems usually form an integral functional element to ensure that the cleaning results can be reproduced easily and in compliance with the strictest hygiene standards.
THIS COULD ALSO BE INTERESTING FOR YOU
